Abstract
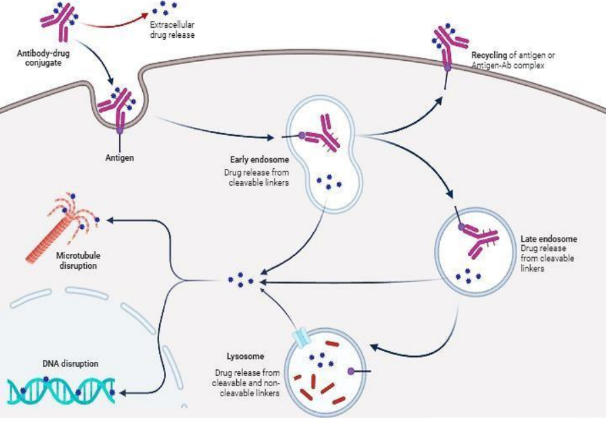
Conjugated antibody drugs constitute an innovative class of biopharmaceuticals that combines the remarkable specificity of monoclonal antibodies with the high potency of small molecule drugs. This review article offers a comprehensive overview of ADCs, from historical origins to clinical application and future development of this class of drugs. We consider in detail the structural elements of ADCs: including antibody choice, linker technologies, and payload optimization. Advancements in mechanism of action include targeted delivery, internalization, and controlled release of cytotoxic agents. Focus will be on applications of ADCs in cancer therapy-including FDA approved ADCs and promising candidates in clinical trials. The horizon of ADCs for expanding applications in autoimmune and infectious diseases, amongst others, will also be addressed. This chapter covers recent technological advancements in ADC design, such as site-specific conjugation strategies and novel payload classes. We discuss challenges that represent a roadblock in ADC development, including resistance mechanisms, how potential toxicity could be more effectively managed, and the potential of combination therapies. We also briefly outline future directions and then underscore personalized medicine approaches that could best realize the therapeutic potential of ADCs. This will actually give an all-inclusive analysis, from which researchers, clinicians, and pharmaceutical professionals can be abreast of the new field that is quickly emerging, antibody-drug conjugates.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright (c) 2025 Oral Sphere Journal of Dental and Health Sciences